- Home
- Services
- Branch iO API Integration Services
- Socket iO API Integration Services
- Mailchimp API Integration Services
- SurveyMonkey API Integration Services
- SendGrid API Integration Services
- ID Merit API Integration Services
- Firebase API Integration Services
- Social Media API Integration Services
- Payment Gateway API Integration Services
- Google API Integration Services
- Industries
- hire developers
- About Us
- Career
- Case Study
Web Application for Clinical Research Organization
Clinical Research
Web Application for Clinical Research Organization
ACRO India founded and registered in India in Feb 2005 has been formed under the aegis of Confederation of Indian Industry (CII) for bringing all CRO’s operating in India on one common platform. ACRO will be used to promote quality research, uphold ethics, share best practices, promote synergies amongst members, to deliberate and act upon common concerns,specially with regards to Indian regulations and industry environment. ACRO will represent the interests of Indian CROs to regional, national and international authorities and organizations.
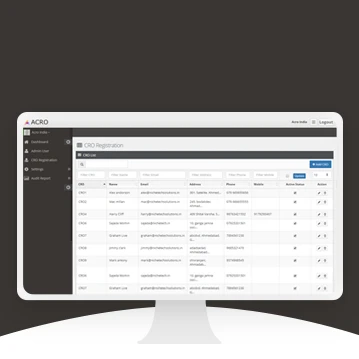




Key problems
-
To fulfil erected need of maintaining volunteer registration and participation across all CROs.
-
Main purpose was to prevent cross participation of any volunteer in more than one CRO, hence maintaining integrity of research.
-
Prevent volunteer to participate in other research for specific period of time after last successful participation for their own benefits.
-
Regulatory authority can audit one place for all the research happening across India.
solution
-
Web-based application for data hosting by all participating CROs by providing volunteer fingerprints,DOB, Initials, etc. which will form the basis of identification of the volunteer.
-
Volunteer apply for screening using fingerprints.
BENEFITS REALIZED
-
Multiple participation & cross participation prevented.
-
Participants health care and access new treatments before they are widely available.
-
Hierarchy of multiple roles and each role equipped with specific task and authority.
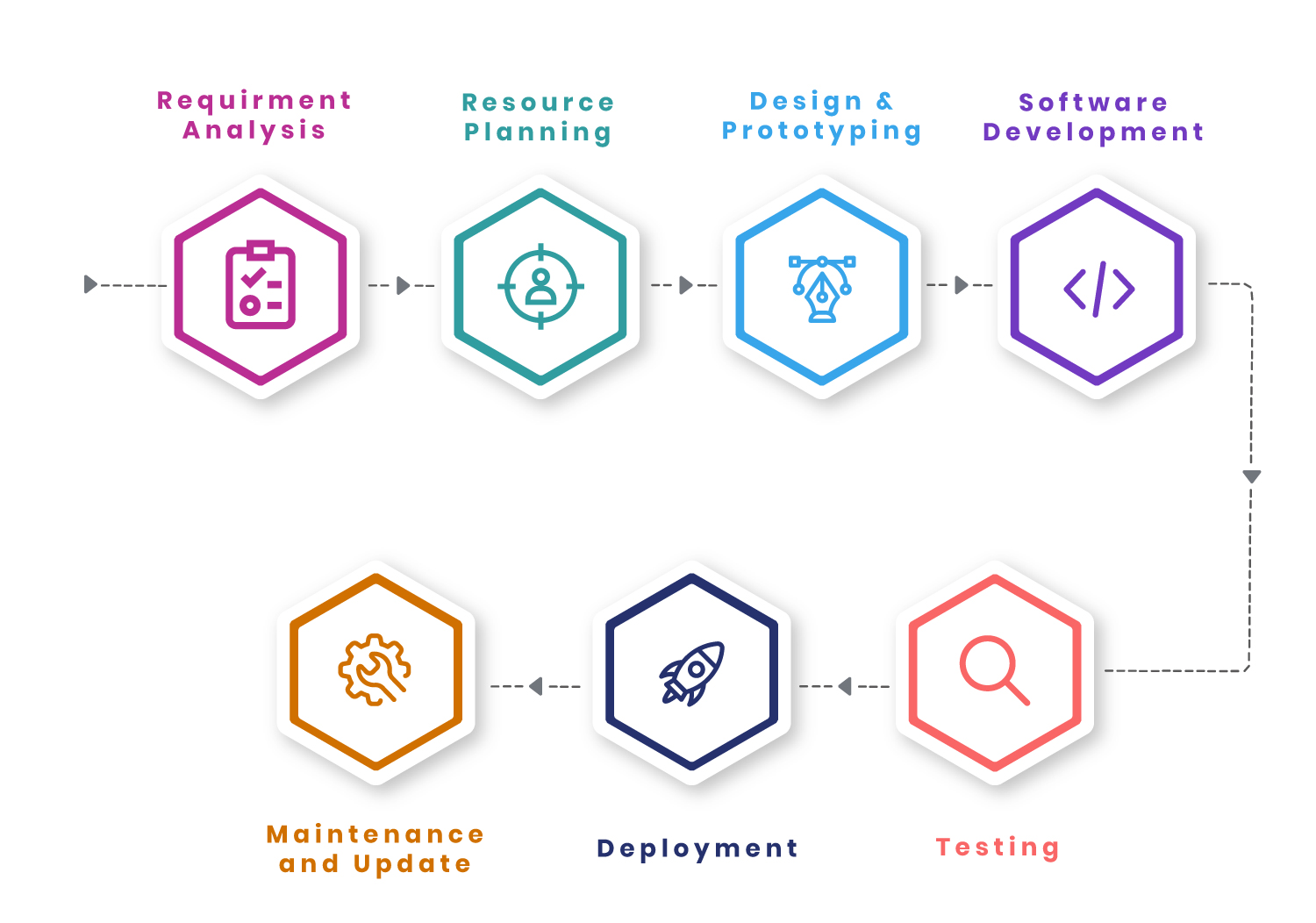
Development Process We Follow
Our design process follows a proven approach. We begin with a deep understanding of your needs and create a planning template.
More case studies

Pharma Manfucturer
Pharmacy Contract Manufacturer
Content Management System and Quote Builder App
Pinestar Inc USA
 Nichetech Bot
Nichetech Bot
 Your experience on this site will be improved by allowing cookies.
Your experience on this site will be improved by allowing cookies.